Raising target to $8
OKYO PHARMA LIMITED
Poised to Reach Our New $8 Price Target
Rob Goldman
June 11, 2025
OKYO PHARMA LIMITED (NASDAQ – OKYO – $2.07)
Industry: BioPharma
6-12 Mo. Price Target: $5.00
COMPANY SNAPSHOT
OKYO Pharma Limited Is a clinical stage bio-pharmaceutical company developing innovative therapies for the treatment of neuropathic corneal pain (NCP) and dry eye disease (DED), with ordinary shares listed for trading on the NASDAQ Capital Market. OKYO’s lead drug candidate urcosimod successfully completed a 240-patient Phase 2 trial in DED patients and recently completed a 17-patient Phase 2 trial in NCP patients.
KEY STATISTICS
- Price as of 6/10/25$2.07
- 52 Wk High – Low$2.13 – $0.8080
- Est. Shares Outstanding34.0M
- Market Capitalization$70.4M
- Average Volume125,120
- Exchange:NASDAQ
COMPANY INFORMATION
OKYO Pharma Limited
14-15 Conduit Street
London W1S 2XJ
United Kingdom
Web: www.OKYOPharma.com
Email: info@okyopharma.com
Phone : 917.497.7560
INVESTMENT HIGHLIGHTS
Since our January 2025 report, OKYO’s shares and average daily volume have essentially doubled, due to key fundamental drivers. With potentially favorable milestones ahead in 3Q25, we believe these shares could reach our new $8.00 price target.
In April 2025, OKYO elected to conclude its Phase 2 NCP trial early and top-line results are expected to be released by mid-3Q25. OKYO is the first company to be granted an investigational new drug (IND) application for NCP by FDA for clinical trials in NCP patients, and the first company to launch and conclude a clinical trial in NCP patients specifically diagnosed with NCP. This trial is on the heels of a favorable DED Phase 2 trial of OK-101 which notably demonstrated statistical significance in an ocular pain secondary endpoint.
The potential size of the NCP market is in the billions and given the debilitating effects of the condition, plus the recently-granted Fast Track designation by the FDA for urcosimod to treat NCP patients, there is considerable interest being generated in this program.
We believe that given its positioning, and assuming data are favorable, OKYO will likely also attract a potential joint venture development partner or an acquirer. We believe the results from this trial represent a major binary event, serving as a critical catalyst for a re-valuation of the stock.
OKYO: WIND AT ITS BACK
Since our January 2025 company report, OKYO Pharma Limited (NASDAQ – OKYO) has been a stellar performer, nearly doubling its share price while achieving a new 52-week high earlier this week. Moreover, these shares have enjoyed a commensurate doubling of its average daily volume, led by a series of recent milestones that we outlined in our previous report. With potentially ground-breaking developmental news on the horizon during 3Q25, we believe that these shares have just begun their ascent toward our new, $8.00 price target.
OKYO may have discovered a first-of-its-kind treatment for a debilitating ocular pain condition called neuropathic corneal pain (NCP) that has no FDA approved, effective treatment. NCP is a debilitating condition affecting many tens of thousands worldwide, and is characterized by chronic, severe eye discomfort. A Phase 2 clinical trial for NCP commenced in October 2024 with top-line results slated to be released during 3Q25, an accelerated timeline from the original end of year estimate.
Urcosimod, the recently assigned official name by USAN for OKYO’s lead candidate, OK-101, was developed using a membrane-anchored-peptide (MAP) technology to produce a novel long-acting drug candidate for treating ocular front-of-the-eye diseases.
Urcosimod is a lipid conjugated chemerin peptide agonist of the ChemR23 G-protein coupled receptor which is typically found on immune cells of the eye and neurons and glial cells found on the dorsal root ganglion. ChemR23 is generally recognized as being responsible for the inflammatory response. Urcosimod was shown to produce anti-inflammatory and pain-reducing activities in mouse models of both dry eye disease and a neuropathic corneal pain animal model; and is designed to combat washout through the inclusion of the lipid ‘anchor’ contained in the candidate drug molecule to enhance the residence time of urcosimod within the ocular environment.
We estimate that the potential size of the market could be $6.4 billion or greater.
Recent Events have Served as Fundamental Drivers
In March 2025, OKYO filed a Fast Track designation application with the FDA for urcosimod, and on May 1, 2025, OKYO announced the FDA has granted Fast Track designation for urcosimod to treat NCP. Receipt of this designation typically leads to accelerating further clinical development as turn-around time for meetings with the FDA is shortened, as well as the potential for a rolling FDA review of the New Drug Application (NDA) to treat NCP.
On March 31, 2025, OKYO announced successfully establishing that urcosimod has been shown to be stable for over two and a half years in single-use ampoules used for administration of the drug to patients. Achieving this objective is a key, core component in meeting CMC (Chemistry, Manufacturing and Controls) requirements for a successful New Drug Application with the FDA. The FDA requires long-term shelf life of drugs as part of the approval process.
Early Closure of Phase 2 Trial a Major Plus
In late April 2025, OKYO reached its most significant step to date with respect to the urcosimod Phase 2 trial designed to treat 48 patients with NCP. After the completion of 17 patients of the intended 48 patients designed for the study, OKYO leadership elected to close the trial early to enable initiating analysis of the clinical data. This decision to close the trial was driven by OKYO’s strong desire to access the currently masked data in the randomized, placebo-controlled trial, and to use it to start planning further development of urcoisimod, assuming the top-line data are positive. Notably, OKYO pointed out that patients participating in the trial have all been diagnosed with long term chronic neuropathic corneal pain and had previously been treated with multiple therapies with very limited or no response.
Significant interest was also reported to be seen in the trial from long-term sufferers of NCP, and OKYO believed it should plan to expand the development program and move forward with a multicenter trial.
The implications of the early trial completion plus desire to immediately analyze clinical results and release top-line data suggest two immediate outcomes, pending positive top-line results, in our view. One, it has been reported by OKYO that a considerable number of potential patients have expressed an interest in participating in a future registrational trial. Two, a number of patients also have requested compassionate use of urcosimod which OKYO announced it is seeking to arrange with Tufts Medical Center, subject to the necessary FDA consents. While this is not a demonstration of evidentiary efficacy of urcosimod, it is a compelling set of observations that suggests a potential drug effect and illustrates the challenging condition in which the patients suffer and the need for the Fast Track designation by the FDA.
LOOKING AHEAD
Management believes that concluding the trial early enables OKYO to save time in advancing the program to a meeting with FDA to discuss further development of urcosimod. Moreover, the analysis of the data from the 17 patients in the study could lead to the release of top-line results in the first half of 3Q25. Considering that urcosimod has already demonstrated favorable safety and placebo-like tolerability in OKYO’s previous 240 patient Phase 2 trial, we believe that OKYO leadership has reasons to be encouraged.
If top-line results are favorable when announced in the next quarter, the Company would likely seek to request an end-of-phase 2 meeting with FDA to explore accelerated plans for the drug’s further clinical development. Topics would likely include future trial design and protocols, primary and secondary endpoints, along with subject size and the number of sites. At this stage it is too early to speculate on the timing and roadmap of future development. However, the key takeaway for investors is that the acceleration of the release of potentially favorable top-line Phase 2 data, assuming the data are positive, should shorten the time that we believe these shares would take to reach our upgraded, $8.00 price target.
Against this backdrop, if an updated roadmap is announced due to favorable data, timelines, and prospective outcomes bode well for OKYO.
Our original $5.00 price target reflected a longer Phase 2 trial that would lead to later published results. However, we have elected to raise our price target to reflect a change in status, in our view. These changes include the acceleration of the closing of the trial, the desire of some patients to utilize the drug on a compassionate basis, and the Fast Track designation. These are all new events since our January 2025 report. As a first-mover that has the potential to publish favorable results for a debilitating condition that has a serious unmet need, and represents a multi-billion-dollar market, we believe these shares could reach the $8 level or higher, in short order. Furthermore, we believe that given the M&A activity in the ocular space, a deal with a partner could occur, and that the Company would benefit from the aforementioned re-valuation of these shares.
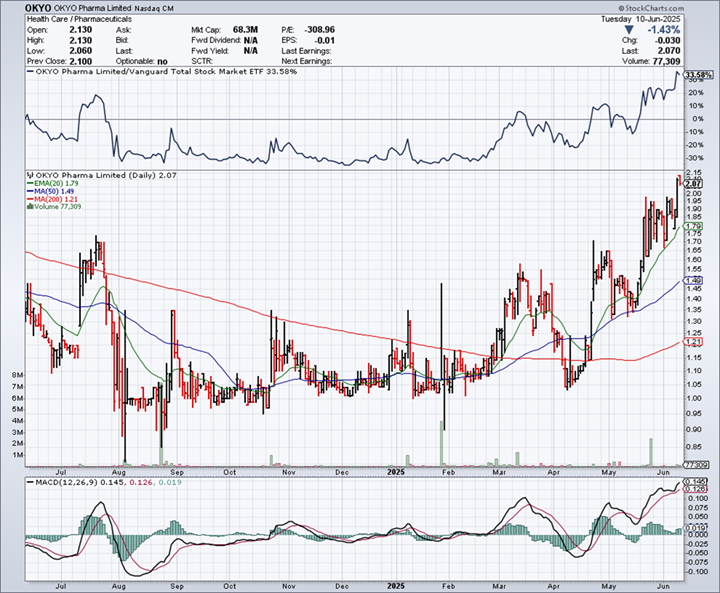
RECENT TRADING HISTORY FOR OKYO
(Source: www.StockCharts.com)
Senior Analyst: Robert Goldman
Rob Goldman founded Goldman Small Cap Research in 2009 and has over 25 years of investment and company research experience as a senior research analyst and as a portfolio and mutual fund manager. During his tenure as a sell side analyst, Rob was a senior member of Piper Jaffray’s Technology and Communications teams. Prior to joining Piper, Rob led Josephthal & Co.’s Washington-based Emerging Growth Research Group. In addition to his sell-side experience Rob served as Chief Investment Officer of a boutique investment management firm and Blue and White Investment Management, where he managed Small Cap Growth portfolios and The Blue and White Fund.
Analyst Certification
I, Robert Goldman, hereby certify that the view expressed in this research report accurately reflect my personal views about the subject securities and issuers. I also certify that no part of my compensation was, is, or will be, directly or indirectly, related to the recommendations or views expressed in this research report.
Disclaimer
This Opportunity Research report was prepared for informational purposes only.
Goldman Small Cap Research, (a division of Two Triangle Consulting Group, LLC) produces research via two formats: Goldman Select Research and Goldman Opportunity Research. The Select format reflects the Firm’s internally generated stock ideas along with economic and stock market outlooks. Opportunity Research reports, updates and Microcap Hot Topics articles reflect sponsored (paid) research but can also include non-sponsored micro-cap research ideas that typically carry greater risks than those stocks covered in the Select Research category. It is important to note that while we may track performance separately, we utilize many of the same coverage criteria in determining coverage of all stocks in both research formats. Research reports on profiled stocks in the Opportunity Research format typically have a higher risk profile and may offer greater upside. Goldman Small Cap Research was compensated by the Company in the amount of $4000 for a research report production and distribution, including a press release. In 2023, Goldman Small Cap Research was compensated by a third party (TraDigital Marketing Group, Inc.) in the amount of $4000 for a research report production and distribution, including a press release. All information contained in this report was provided by the Company via filings, press releases or its website, or through our own due diligence. Our analysts are responsible only to the public, and are paid in advance to eliminate pecuniary interests, retain editorial control, and ensure independence. Analysts are compensated on a per report basis and not on the basis of his/her recommendations.
Goldman Small Cap Research is not affiliated in any way with Goldman Sachs & Co.
Separate from the factual content of our articles about the Company, we may from time to time include our own opinions about the Company, its business, markets and opportunities. Any opinions we may offer about the Company are solely our own and are made in reliance upon our rights under the First Amendment to the U.S. Constitution, and are provided solely for the general opinionated discussion of our readers. Our opinions should not be considered to be complete, precise, accurate, or current investment advice. Such information and the opinions expressed are subject to change without notice.
The information used and statements of fact made have been obtained from sources considered reliable but we neither guarantee nor represent the completeness or accuracy. Goldman Small Cap Research did not make an independent investigation or inquiry as to the accuracy of any information provided by the Company, or other firms. Goldman Small Cap Research relied solely upon information provided by the Company through its filings, press releases, presentations, and through its own internal due diligence for accuracy and completeness. Such information and the opinions expressed are subject to change without notice. A Goldman Small Cap Research report or note is not intended as an offering, recommendation, or a solicitation of an offer to buy or sell the securities mentioned or discussed. This report does not take into account the investment objectives, financial situation, or particular needs of any particular person. This report does not provide all information material to an investor’s decision about whether or not to make any investment. Any discussion of risks in this presentation is not a disclosure of all risks or a complete discussion of the risks mentioned. Neither Goldman Small Cap Research, nor its parent, is registered as a securities broker-dealer or an investment adviser with FINRA, the U.S. Securities and Exchange Commission or with any state securities regulatory authority.
ALL INFORMATION IN THIS REPORT IS PROVIDED “AS IS” WITHOUT WARRANTIES, EXPRESSED OR IMPLIED, OR REPRESENTATIONS OF ANY KIND. TO THE FULLEST EXTENT PERMISSIBLE UNDER APPLICABLE LAW, TWO TRIANGLE CONSULTING GROUP, LLC WILL NOT BE LIABLE FOR THE QUALITY, ACCURACY, COMPLETENESS, RELIABILITY OR TIMELINESS OF THIS INFORMATION, OR FOR ANY DIRECT, INDIRECT, CONSEQUENTIAL, INCIDENTAL, SPECIAL OR PUNITIVE DAMAGES THAT MAY ARISE OUT OF THE USE OF THIS INFORMATION BY YOU OR ANYONE ELSE (INCLUDING, BUT NOT LIMITED TO, LOST PROFITS, LOSS OF OPPORTUNITIES, TRADING LOSSES, AND DAMAGES THAT MAY RESULT FROM ANY INACCURACY OR INCOMPLETENESS OF THIS INFORMATION). TO THE FULLEST EXTENT PERMITTED BY LAW, TWO TRIANGLE CONSULTING GROUP, LLC WILL NOT BE LIABLE TO YOU OR ANYONE ELSE UNDER ANY TORT, CONTRACT, NEGLIGENCE, STRICT LIABILITY, PRODUCTS LIABILITY, OR OTHER THEORY WITH RESPECT TO THIS PRESENTATION OF INFORMATION.
For more information, visit our Disclaimer: www.goldmanresearch.com
